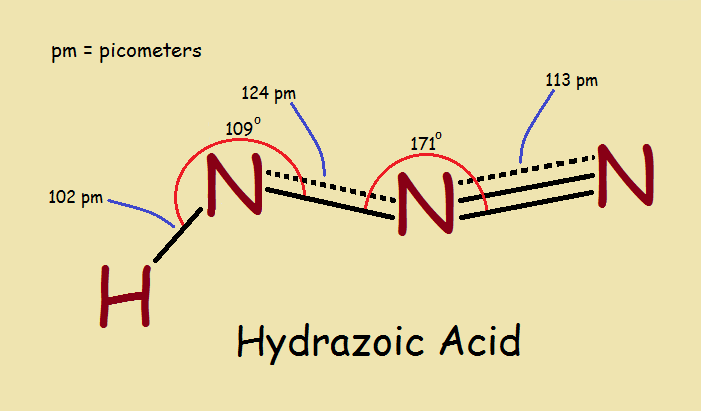


When we think of nitrogen and hydrogen, we are likely to think first of ammonia, NH3 . But what of hydrazoic acid? Hydrazoic acid is HN3 . It looks to be the opposite of ammonia. What is the chemical structure of hydrazoic acid and its azide derivatives? And why are they of importance to us?
When we think of hydrogen, we think of a +1 valence, though at times it is assigned a –1 valence. Similarly, nitrogen ordinarily assumes either a +3 or a +5 valence. Both ammonia and hydrazoic acid exhibit a +3 valence for nitrogen.
All of this is well and good, but what is the structure of ammonia? of hydrazoic acid and the azides? In both instances, we may be surprised that what we imagine may not be correct! Note the series of illustrations, below…
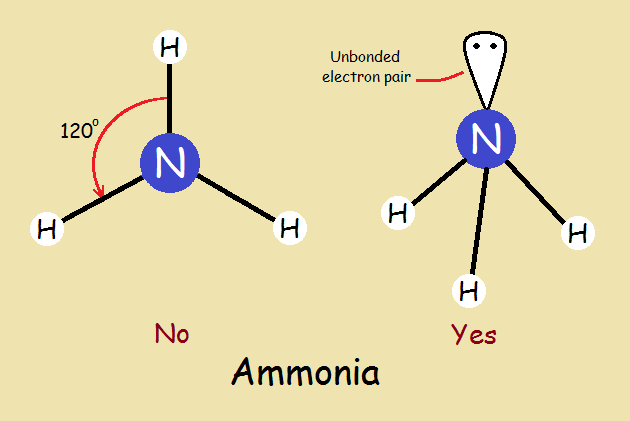
We note that a molecule of ammonia more closely resembles a tripod than it does a propeller. This is due to an unbonded pair of electrons and the molecule’s trigonal pyramidal shape. How well this illustrates the wisdom in not being too quick to “assume”.
With the chemical formula HN3 , what would you anticipate a molecule of hydrazoic acid would look like. It might seem to be a 3-member nitrogen ring structure bonded to a lone hydrogen atom, as in the image below, right? That would be an intelligent deduction, but uninformed?
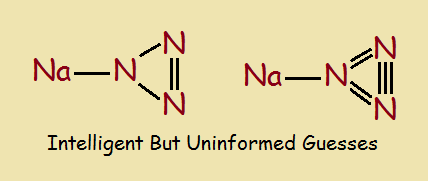
Spectroscopic and other tests can be used to reveal chemical structure. Once that is done, the results are seen to be entirely different from the “intelligent guess”. Why, there is no ring in the structure! The correct structure is the main image associated with this article.
Azide bond lengths point to a need to look for resonance structures. These do, indeed, exist. There are two¹ that catch the eye of the informed chemist, as drawn below…

In reality, such resonance structures often suggest an actual structure intermediate between the contributing structures. One bond between a single and a double bond plus the other bond between a double and a triple bond suggest two bonds. One approximately a bond-and-a-half, the other a double-bond-and-a-half.
There are uses for the azides. Often they take advantage of their explosive properties. For instance, sodium azide is used in the manufacture of automobile air bags. NaN3 provides the sudden expansion needed to inflate the bags and save potential victims’ lives.
¹ A third resonance structure can be drawn. However it is considered inconsequential, not exerting noticeable influence on the bond lengths and angles of the actual structure. The following is a very brief Lewis Structure video that discusses the point.